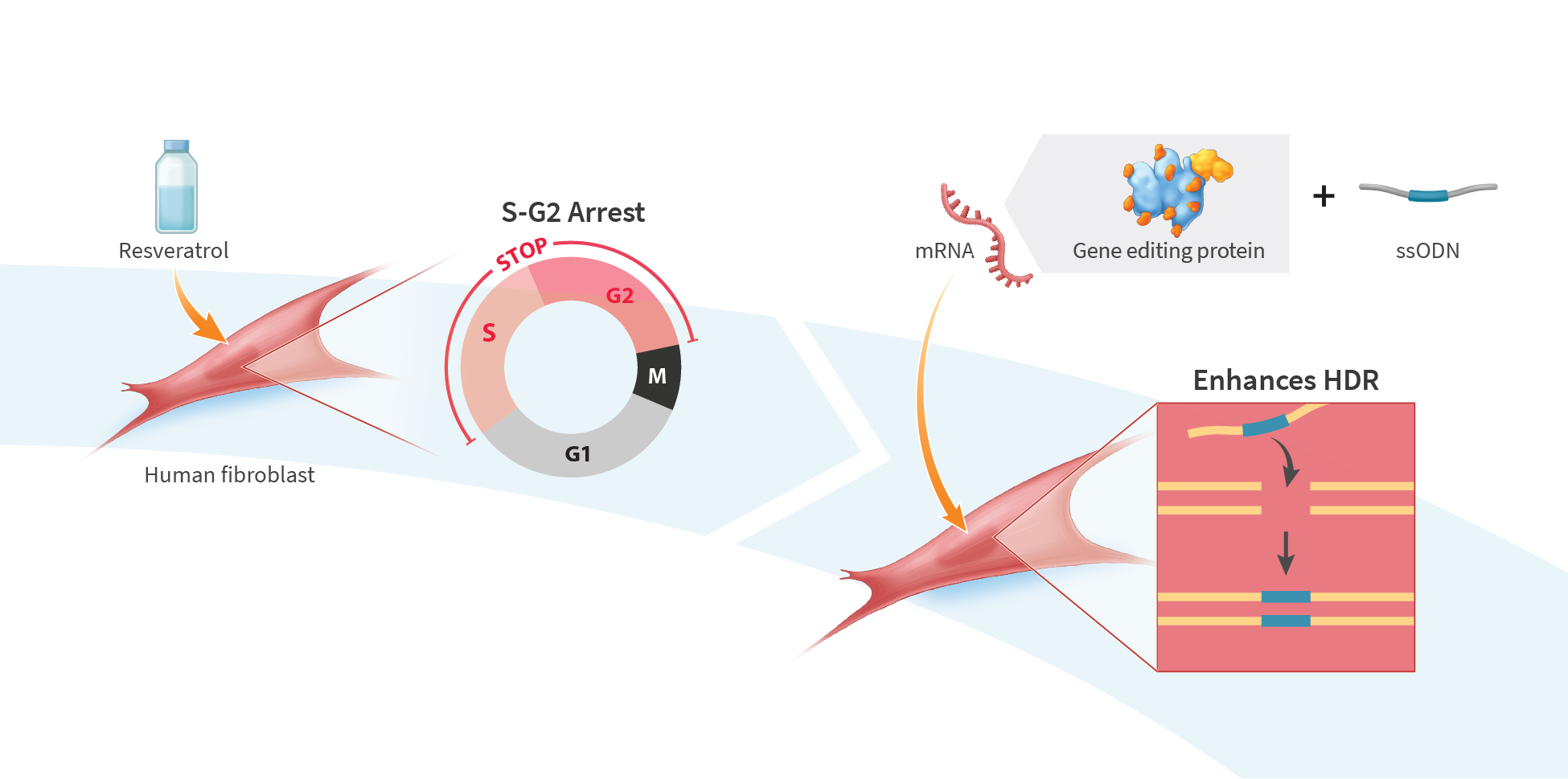
Resveratrol Treatment Increases Homology-Directed Repair in Primary Human Cells
Gene editing technology, which enables the precision modification of DNA in living cells, is being developed for the treatment of various diseases, including genetic diseases and cancer. Gene editing commonly employs sequence-specific endonucleases to create double strand breaks in genomic DNA, and relies on the cell’s DNA repair mechanisms to apply the desired changes. Precise sequence modifications, such as single-base changes, rely on the homology directed repair (HDR) mechanism. Despite its essential role in gene repair, HDR occurs at a very low frequency in many cells compared to other repair mechanisms. Here, we evaluate the impact of resveratrol, a small molecule extracted from grape skin that has recently been shown to promote the expression of key HDR factors and induce cell cycle arrest at S phase in porcine fetal fibroblasts, on single-base editing efficiency in primary human fibroblasts. Following treatment with resveratrol, fibroblasts were co-transfected with mRNA encoding a chromatin context-sensitive gene-editing protein targeting the AAVS1 safe-harbor locus and a single-stranded DNA repair template designed to introduce a SfoI restriction-enzyme site through a G-to-C mutation. Single-base editing efficiency was determined by restriction fragment length polymorphism (RFLP) analysis. Resveratrol treatment prior to transfection increased the S and G2-phase population 2.3-fold and increased HDR efficiency 2-fold compared to untreated cells. Application of resveratrol after transfection (i.e., no cell cycle synchronization) yielded further improvement in single-base editing efficiency (> 2-fold), suggesting that the effects of resveratrol on HDR are not confined to cell-cycle control. Resveratrol treatment provides a straightforward method for improving HDR efficiency in primary human fibroblasts, and may serve as a useful tool in the development of HDR-based gene-editing therapies.