Directed Differentiation of Gene Edited iPSCs by Small-Molecule Inhibition of a Transgene-Encoded Protein
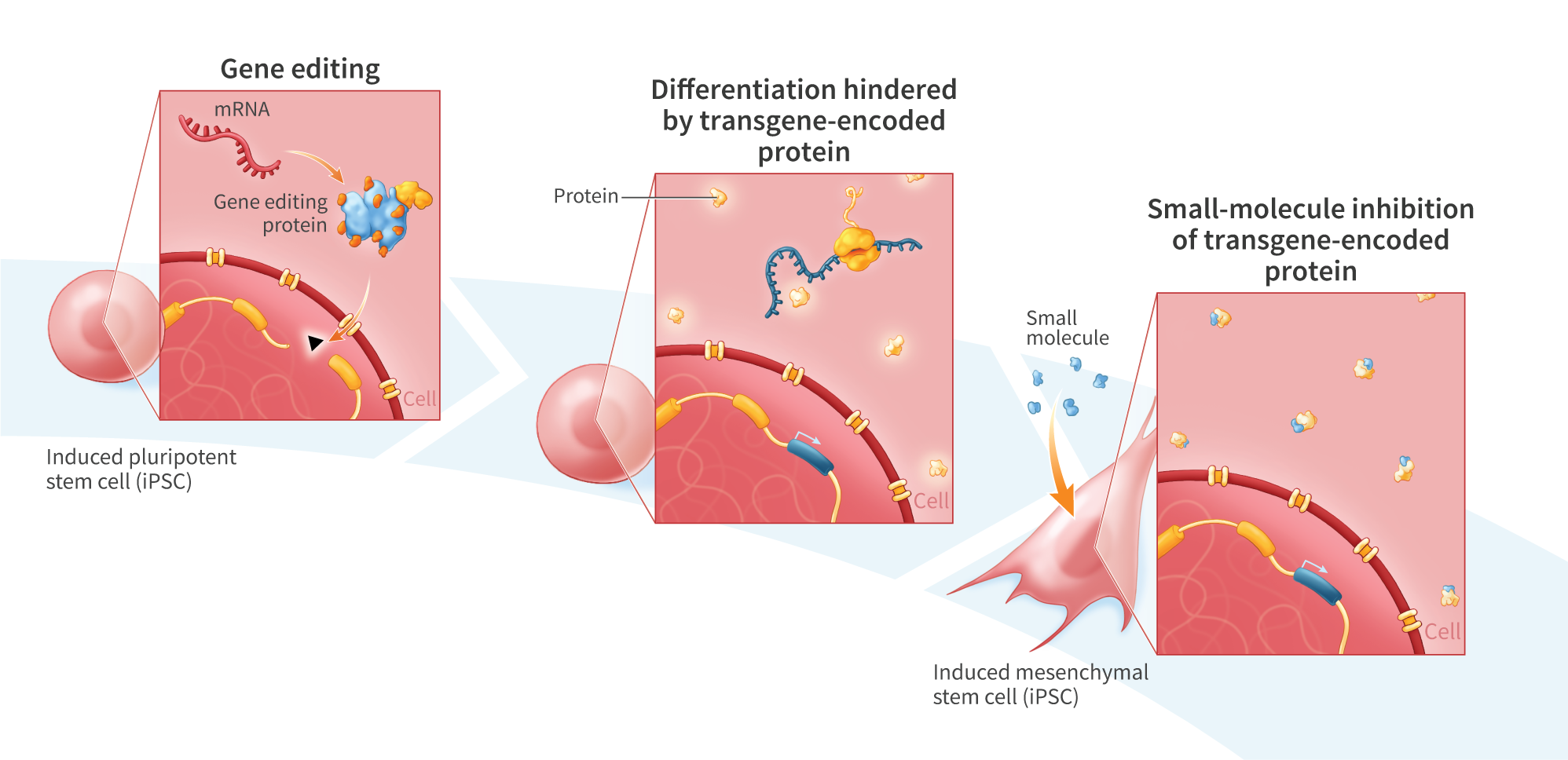
Indoleamine 2,3-dioxygenase 1 (IDO1) is an inducible, heme-containing enzyme that is critically involved in tryptophan catabolism and known to be a prominent immune regulator. Cell therapies with increased IDO1 expression are of high interest for a variety of indications, including autoimmune disorders, inflammatory diseases, transplant recovery, and wound healing. In particular, iPSC-derived mesenchymal stem cells (iMSCs) engineered to overexpress IDO1 may be ideal for suppressing dysregulated immune cells while simultaneously promoting expansion of regulatory T lymphocytes and the M1 (pro- inflammatory) to M2 (anti-inflammatory) polarization of macrophages. Here, we report the development of an iMSC cell line containing an IDO1 transgene under the control of a JeT promoter that was inserted into the AAVS1 safe-harbor locus in mRNA-reprogrammed iPSCs. A clonal population of edited cells (i.e. IDO1-iPSCs) was isolated using single-cell sorting. Bi- allelic insertion of the IDO1 transgene was confirmed by amplicon sequencing. The IDO1-iPSCs were then differentiated to IDO1-iMSCs. During differentiation, we found that the IDO1-iPSCs showed an unexpected, cuboidal morphology and noticeably decreased proliferation rates relative to control iPSCs, possibly indicating that IDO1 was interfering with the differentiation process. Two small-molecule IDO1 inhibitors, Epacadostat, which binds to holo (heme-bound) IDO1 and inhibits the enzymatic function of the protein and IDO1-IN-5, which binds to apo (heme-dissociated) IDO1 and inhibits IDO1-dependent cell signaling, were added to the culture for the final five days of differentiation. The addition of both IDO1 inhibitors (each ata 10uM concentration) increased the proliferation of the partly differentiated IDO1-iPSCs (6 h reduction in doubling time) with a population doubling time approaching that of control iMSCs (29 h vs. 27 h). While both inhibitors could enhance proliferation independently, Epacadostat alone had a larger effect on population doubling time than the IDO1-IN-5 alone (6 h vs. 2 h reduction in doubling time), suggesting that the anti-inflammatory enzymatic function of IDO1 may be most responsible for decreased proliferation during differentiation of IDO1-iPSCs. Notably, we also found that when administering both IDO1 inhibitors to non-engineered control iMSCs, the population doubling time increased relative to untreated test cultures (34 h versus 27 h), suggesting a possible minimum IDO1 protein level required for optimal iMSC culture. These results suggest that small-molecule inhibition of transgene-encoded proteins may form a key element of directed differentiation process development for knock-in iPS cell lines, and may be useful for the scale-up manufacturing of IDO1-iMSCs in particular.