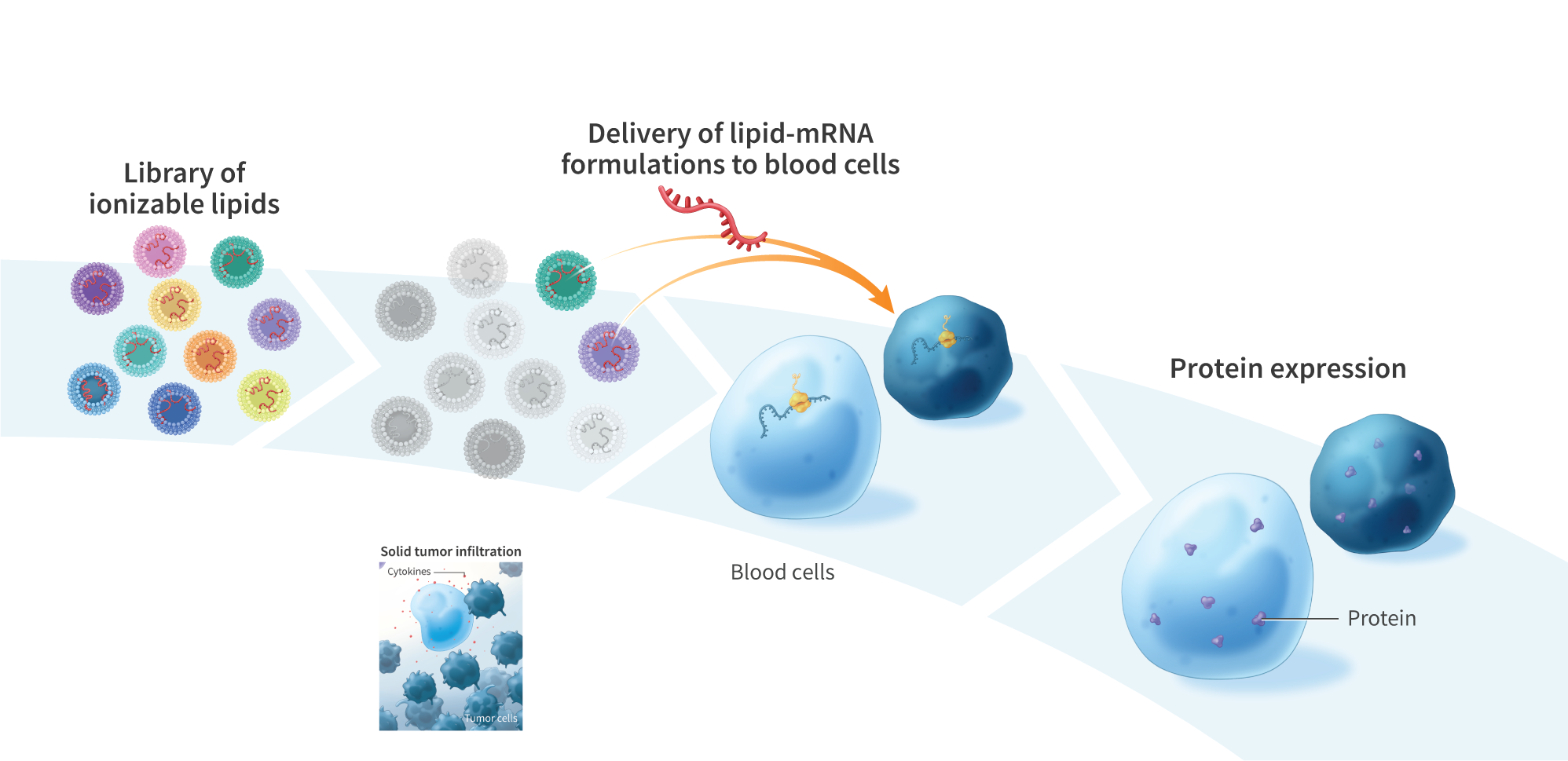
Rational Design of Multivalent Ionizable Lipid Delivery Systems for mRNA Delivery to Blood Cells
In recent years, lipid-based mRNA delivery has become the gold standard method for inducing exogenous protein production in vivo as evidenced by the success of the COVID-19 mRNA vaccines. While intramuscular injections are ideal for vaccine applications, intravenous injections are generally more suitable for achieving broad internal distribution of therapeutic payloads. Following systemic administration, blood cells are the first cells encountered by lipid nanoparticles (LNPs) and therefore serve as high interest targets for in situ protein production. With the goal of identifying a lipid formulation capable of efficiently transfecting blood cells, we synthesized and screened a library of 16 multivalent ionizable lipids with variations in headgroup and lipid tail. Headgroup variations included spermine (a naturally occurring biomolecule), dihydroxyspermine, 2- hydroxypropylamine, and ethanolamine (the headgroup of SM-102, the ionizable lipid component of Spikevax). The lipid tails varied in degree of unsaturation, carbon chain length, and head-to-tail spacer length. Lipids were characterized as lipoplexes and as solid LNPs, using mRNA encoding green fluorescent protein (GFP) as representative nucleic acid cargo. Nanoparticle size, surface charge, mRNA encapsulation, transfection efficiency, and cellular toxicity were evaluated. Lipoplexes were formulated at various weight ratios of lipid/mRNA, and mRNA-loading efficiency was determined via gel electrophoresis. The lowest lipid/mRNA weight ratio that displayed complete complexation for each lipid was used for the corresponding lipids in subsequent in vitro analyses. Notably, the greatest transfection efficiency in the lipoplex form was induced by FB3-54, a lipid comprised of a spermine headgroup and bis hexyl 2-hexyldecanoate tails. FB3-54 lipoplexes enabled efficient transfection of GFP mRNA in the THP-1 human monocyte cell line and exhibited a half-maximal effective concentration (EC50) of 147 ng RNA per 50,000 cells, which was lower than that of Lipofectamine 3000 (242 ng RNA per 50,000 cells). The measured diameter of the FB3- 54 lipoplexes was 359 nm. FB3-54 also functioned effectively as a solid LNP when using a molar ratio of 50:38.5:10:1.5 (ionizable lipid/cholesterol/DSPC/DMG-PEG2000) and a 0.12 weight ratio of mRNA to ionizable lipid. FB3-54 LNPs were 170 nm in diameter, possessed a surface charge of +3.6 mV (at a pH of 7.0), demonstrated near complete encapsulation efficiency, and exhibited greater in vitro transfection efficiency than the clinically used Dlin-MC3-DMA LNPs. The ability to efficiently target blood cells using lipid delivery systems opens the door to a variety of applications, including potent in vivo mRNA delivery and cell reprogramming.