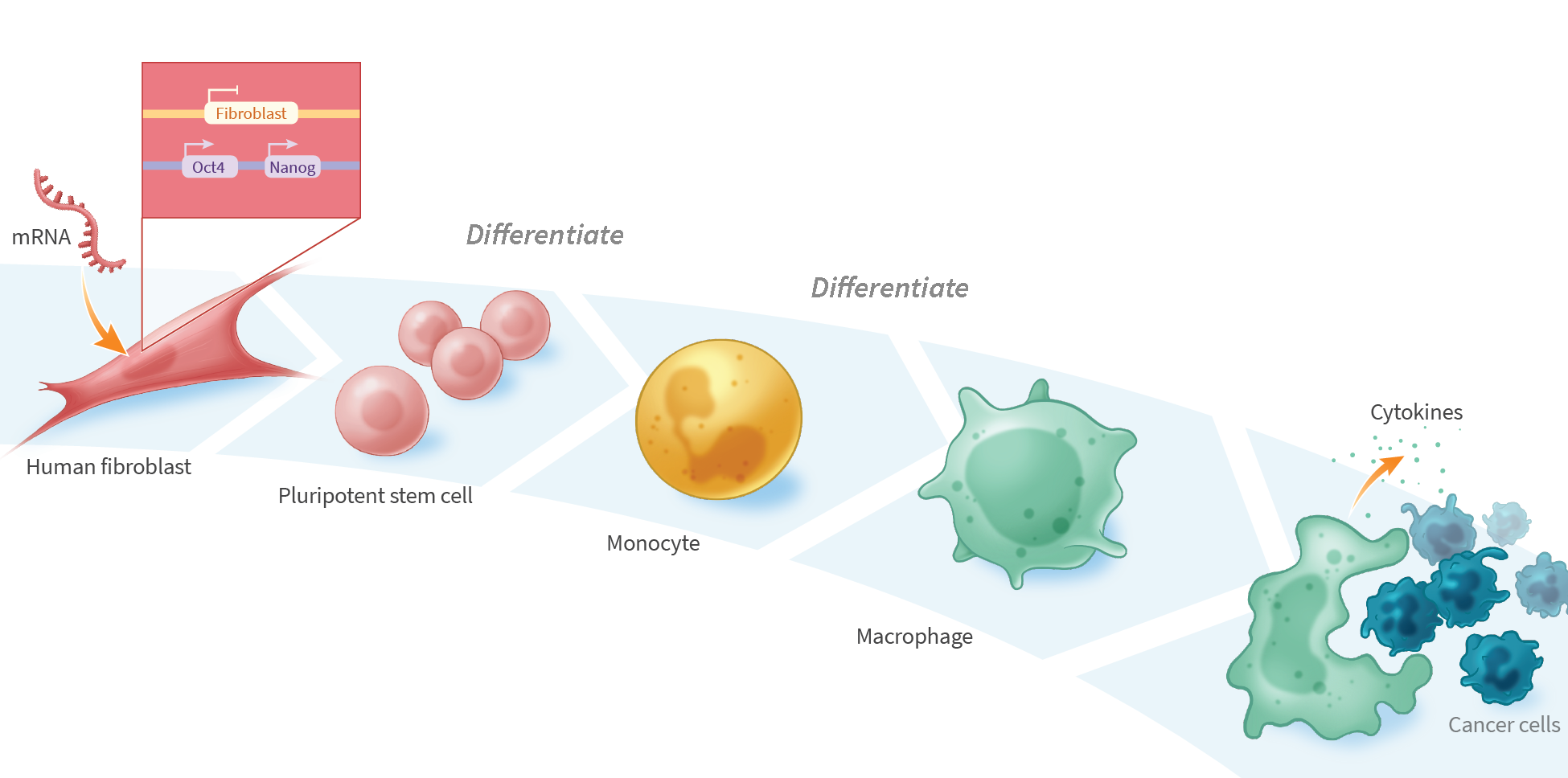
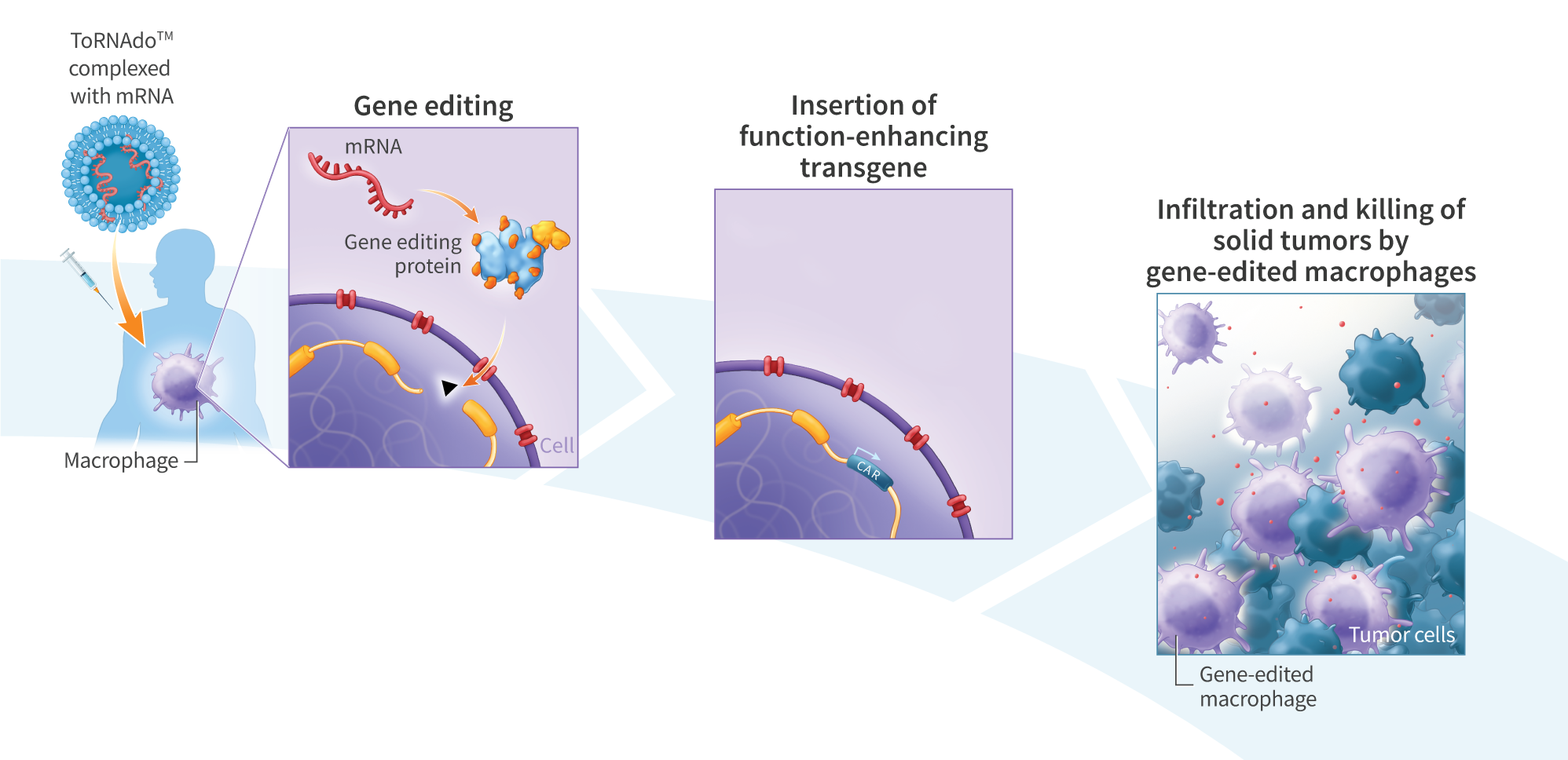
iPSC-Derived Monocytes Generate Functional M1 and M2 Macrophages with Enhanced Cytokine Secretion and Tumor Cell-Killing Activity
Cancer immunotherapy has advanced rapidly over the past two decades, with several autologous chimeric antigen receptor (CAR)-T cell therapies approved for the treatment of hematologic cancers. However, CAR-T cells have shown limited activity against solid tumors, in part due to the immunosuppressive nature of the tumor microenvironment preventing CAR-T cell infiltration. This has led to investigation of other immune cells as alternatives to T-cell-based therapies, including monocytes and monocyte-derived macrophages, which exhibit innate tumor-infiltration properties. We developed a process for differentiating pluripotent stem cells along a myeloid lineage, and generated populations of cells with characteristics of monocytes and M1 and M2 macrophages, including cytokine secretion and tumor cell-killing activity. mRNA-reprogrammed human induced pluripotent stem cells (iPSCs) were differentiated into monocytes using a 28-day monolayer protocol. Beginning on day 14, cells were harvested every 3-4 days. CD14+ isolation yielded >95% CD14+ cells with an average yield of 4.1×104 cells per cm2 per harvest. iPSC-derived monocytes were compared to peripheral blood mononuclear cell (PBMC)-derived monocytes for expression of key hematopoietic and myeloid-lineage markers CD11b, CD14, CD33, CD45, CD80, CD163, CD206, and SIRPα. iPSC-derived monocytes showed similar expression of CD11b, CD14, CD33, CD45, and CD163 compared to PBMC-derived monocytes, and increased expression of markers indicative of an activated state: CD80 and CD206. Compared to PBMC-derived monocytes, iPSC-derived monocytes showed both higher viability in culture and superior recovery from cryopreservation. iPSC-derived monocytes were further differentiated into macrophages by exposure to MCSF for 3-4 days, and were assessed for their ability to polarize, secrete pro- and anti-inflammatory cytokines, and for cytotoxic activity when co-cultured with cancer cells. M1 macrophages were polarized with interferon gamma (IFN-γ, 50 ng/mL) and lipopolysaccharide (LPS, 10 ng/mL) for 48 hours, while M2 macrophages were treated with IL-4 (10 ng/mL) for 48 hours. iPSC-derived monocytes differentiated into macrophages with >90% efficacy, as assessed by cell adherence, morphology, and surface marker expression (CD14, CD45, CD163). M1 and M2 polarized iPSC-derived macrophages secreted similar levels of TNFα, IL-12p70, and IL-10 compared to PBMC-derived macrophages. iPSC-derived macrophages killed 45% of U2OS cancer cells in vitro after 24 hours at an E:T ratio of 5:1. We demonstrate a process for differentiating mRNA-reprogrammed iPSCs into cytotoxic macrophages. The mRNA reprogramming and differentiation processes are virus-free and DNA-free, avoiding any potential risk of vector integration. These results suggest that mRNA-reprogrammed iPSCs may represent a viable source of macrophages for the development of therapies to treat various indications, including solid tumors.