Efficient Transgene Knock-In in Human iPS Cells Combined With Small Molecule-Mediated “On-Switch” Yields Clonal Populations of Engineered Tissue-Specific Cells
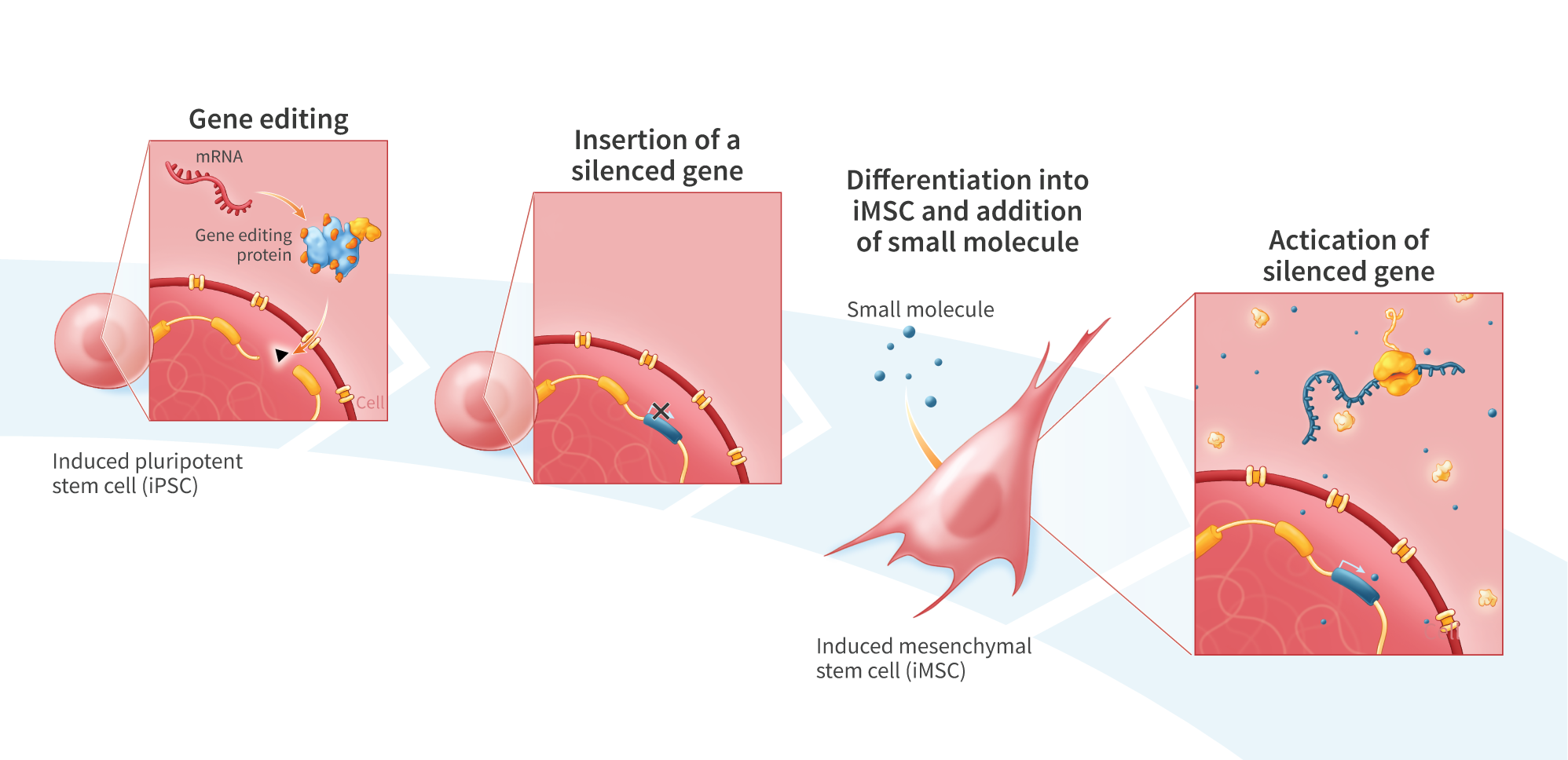
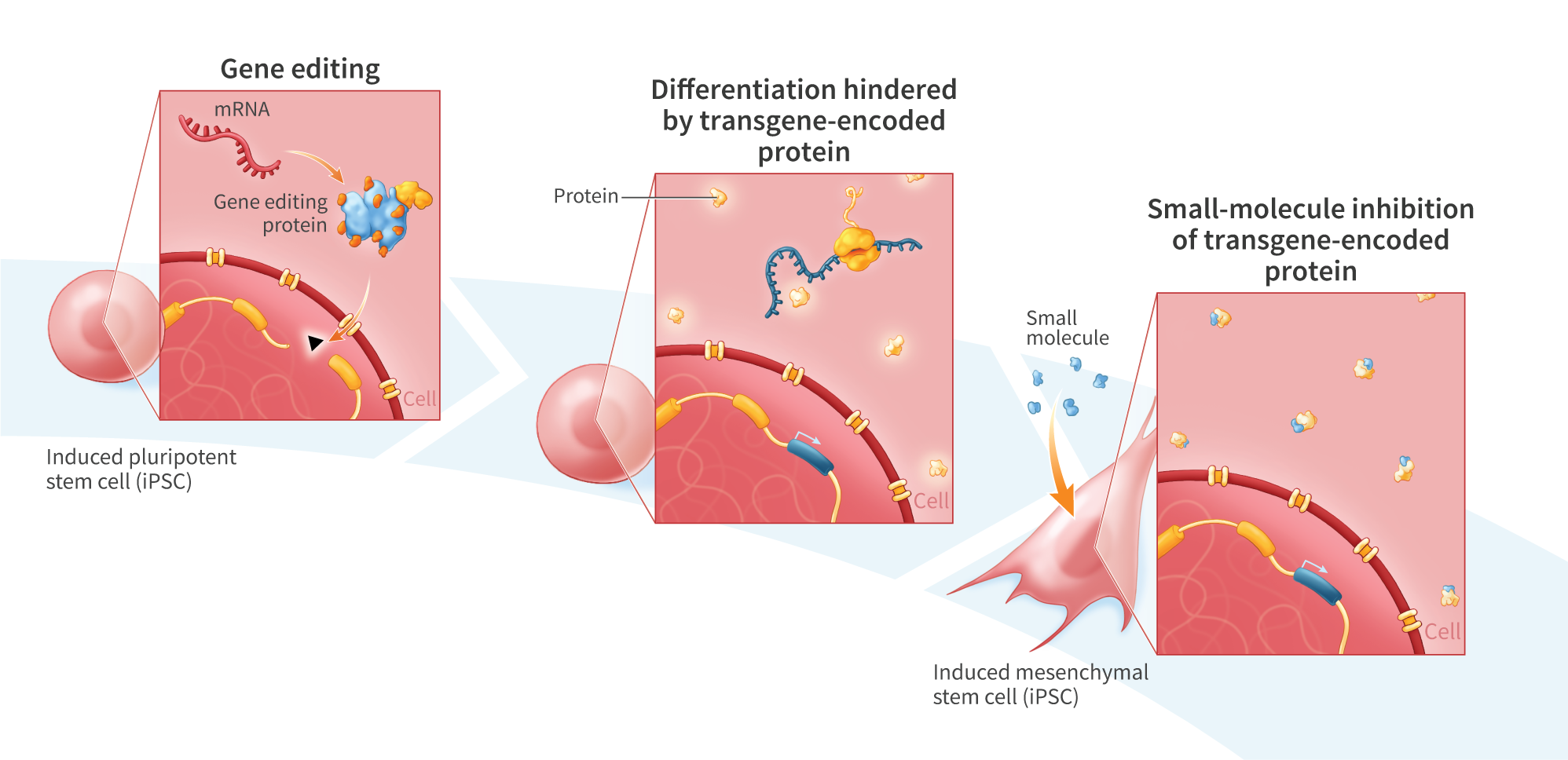
Mesenchymal stem cells (MSCs) are a promising cell-therapy platform with the potential to treat a diverse array of diseases due to their immunomodulator properties – properties which can be enhanced through gene editing. Gene editing autologous or donor- derived MSCs is challenging due to the non-clonal nature of these cell sources, and associated risks of off-target effects. In contrast, iP$ cells, which are clonal and highly expandible, provide an ideal source of cells for gene editing and subsequent differentiation into tissue-specific cells for cell therapy applications. Here we establish stable clonal human induced pluripotent stem (iPS) cell lines engineered to express green fluorescent protein (GFP) using two different promoters. We then differentiate the engineered iPS cells to MSCs (EiMSCs) while monitoring changes in GFP expression, isolate an EiMSC subpopulation and verify differentiation using surface markers. Single-stranded DNA (ssDNA)donors encoding GFP under the control of the JeT or EFla promoters were inserted into iPS cells using mRNA encoding UltraSlice gene-editing proteins targeting the AAVSI safe- harbor locus. GFP transgene insertion rates of 40% and 10% were observed for JeT and EFlapromoter-containing donors, respectively. While EF1a and JeT promoters drive robust GFP expression when inserted directly in iMSCs, in iPS cells strong GFP expression was only observed under EFla – expression was not detected in JeT-GFP iPS cells. Clonal cell lines were generated from both lines using single-cell deposition, and bi-allelic insertion into the AAVSI locus was verified by amplicon sequencing. Engineered iPS cells were differentiated into EiMSCs, and GFP expression was monitored. During differentiation, the number of GFP-expressing cells decreased from >99% in the starting EF1a-GFP iPS cells to 40% in the differentiated EF1a-GFP iMSCs. In contrast, JeT-GFP iPS cells began expressing GFP during differentiation, but stopped expressing GFP near the end of the differentiation process. Treatment with Trichostatin A, a selective histone deacetylase (HDAC) inhibitor, resulted in a temporary increase in GFP expression in JeT-GFP cells during differentiation. To obtain a clonal population of EiMSCs that uniformly express GFP, we then enriched the GFP-expressing EF1a-GFP iMSCs, resulting in a cell line that exhibited traditional MSC surface markers (positive markers: CD90, CD73, CD105, and CD44; negativemarkers:CD34, TRA-1-60, TRA-1-81, CD45, and HLA-DR) and displayed stable GFP expression for over 7 passages. Here we demonstrate a platform for developing clonal EiMSC cell populations that uniformly and stably express a desired protein from a transgene inserted into a defined genomic locus by mRNA gene editing. We also show temporal control of transgene expression using small molecules during directed differentiation of iPS cells. This platform benefits from high knock-in efficiency enabled by mRNA gene editing combined with ssDNA donors and may prove useful for the development of cell therapies engineered to express therapeutic proteins.