mRNA Vectorization of Gene-Editing Proteins
Download our Technology Catalog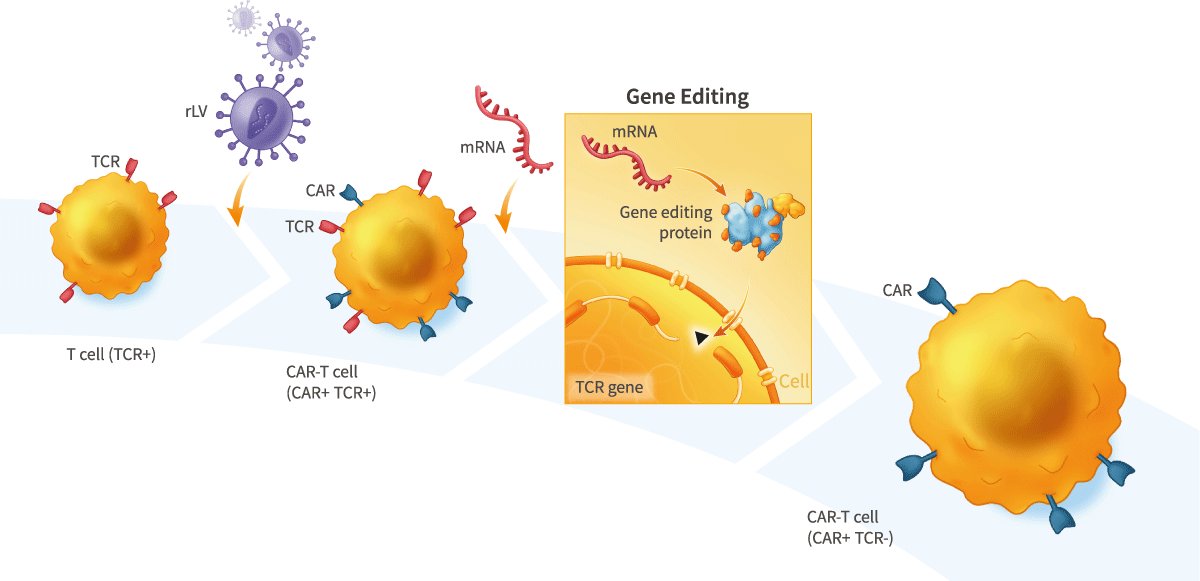
Overview
Gene-editing proteins can be used to inactivate, repair or insert sequences in living cells. Conventional approaches using plasmids or viruses to express gene-editing proteins can result in low-efficiency editing and unwanted mutagenesis when an exogenous nucleic acid fragment is inserted at random locations in the genome.
Our scientists developed a technology that uses mRNA to express gene-editing proteins. This technology can enable dramatically higher efficiency gene editing, including in primary cells, than other approaches, without using viruses or DNA-based vectors that may cause unwanted mutagenesis.
This technology can be used, for example, to generate allogeneic CAR-T therapies for the treatment of cancer in which mRNA encoding gene- editing proteins are used to inactivate the endogenous T-cell receptor to prevent therapeutic T cells from causing graft-versus-host disease (GvHD), and/or to generate allogeneic stem cell-derived therapies in which mRNA encoding gene-editing proteins are used to inactivate one or more components of the human leukocyte antigen (HLA) complex to render the cells immuno-nonreactive or “stealth”.
mRNA Vectorization of Gene-Editing Proteins is protected by three U.S. patents (with additional patents pending in the U.S. and in other countries). Of note, certain granted patents include claims that are not limited by disease indication, cell type, target sequence, mRNA sequence or chemistry, or method of transfection.
Example Applications
- Ultra-high efficiency editing of T cells, fibroblasts, keratinocytes, and pluripotent stem cells
- Ultra-high specificity gene editing
- Virus-free and DNA-free gene editing
- Gene repair using a DNA-repair template
- Donor sequence insertion into a target genomic locus (e.g., TRAC, AAVS1 safe harbor, etc.)
- Gene-editing therapies (ex vivo and in vivo)
- Autologous and allogeneic engineered cell therapies (e.g., CAR-T, CAR-NK, stem cell-derived therapies, etc.)
Data
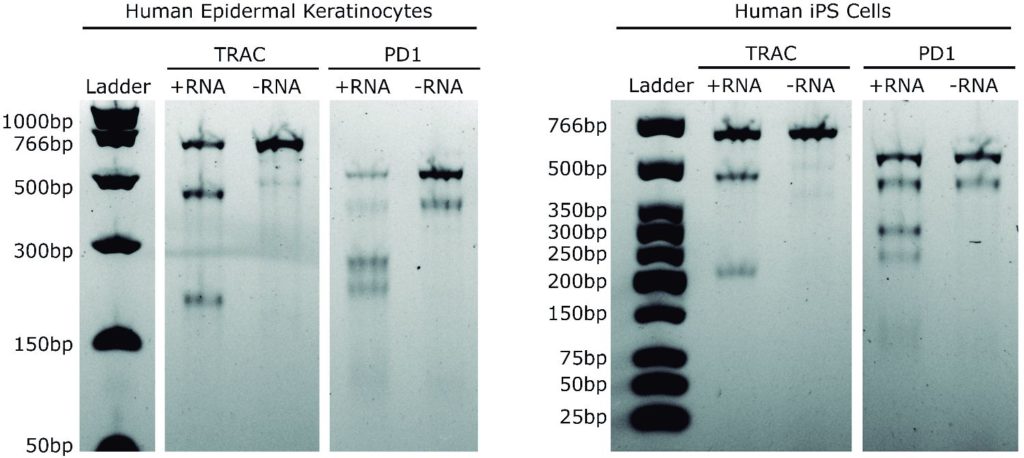
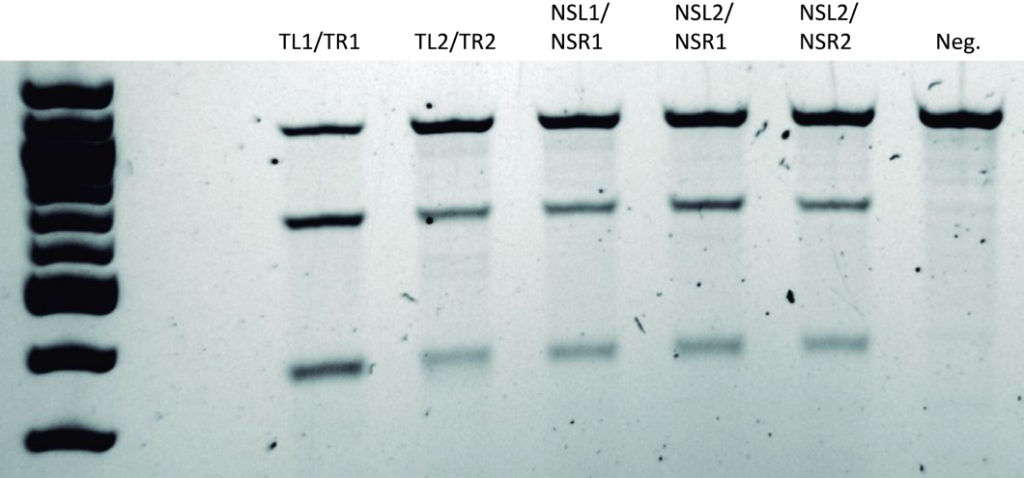
Representative Claim
U.S. Pat. No. 10,662,410
A method for producing a gene-edited cell, comprising:
(a) providing a cell comprising a target DNA sequence;
(b) culturing the cell; and
(c) transfecting the cell with a plurality of synthetic RNA molecules, wherein the synthetic RNA molecules include:
i. a first synthetic RNA molecule encoding a first fusion protein comprising a DNA-binding domain and a catalytic domain of a nuclease; and
ii. a second synthetic RNA molecule encoding a second fusion protein comprising a DNA-binding domain and a catalytic domain of a nuclease;
wherein:
the first fusion protein and the second fusion protein are independently a transcription activator-like effector nuclease (TALEN);
the transfecting results in the cell expressing the first fusion protein and the second fusion protein to result in a double-stranded break in the target DNA sequence; and
the first synthetic RNA molecule and the second synthetic RNA molecule are independently synthesized by in vitro transcription from a DNA template.