Combined mRNA Gene-Editing & Cell Reprogramming
Download our Technology Catalog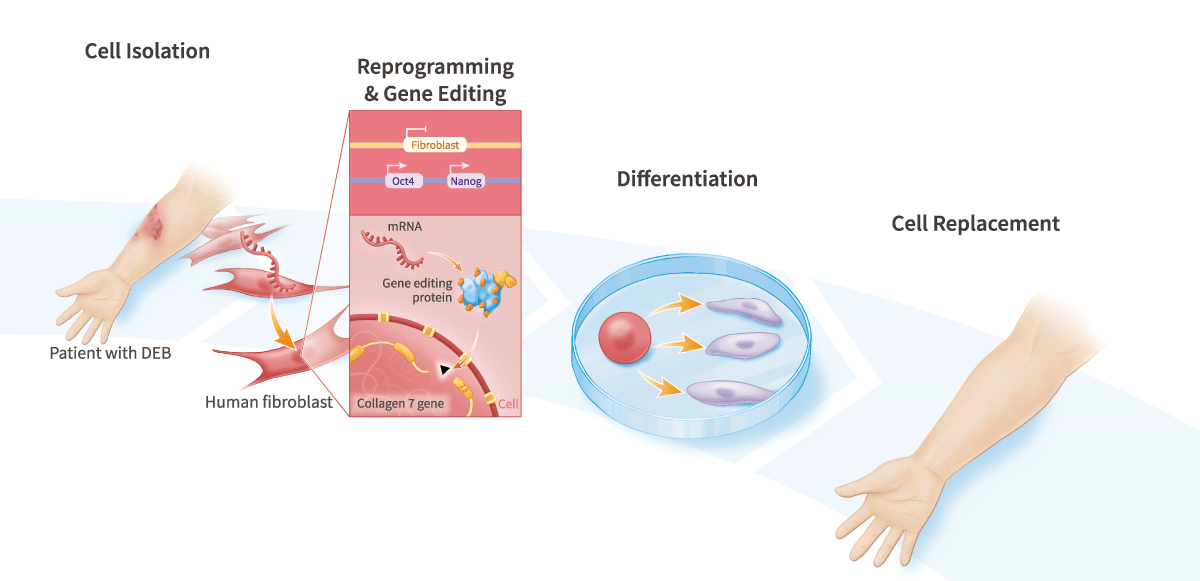
Overview
Combining gene-editing with cell reprogramming enables the generation of gene-corrected personalized cell therapies, models of genetic disease, engineered cell therapies, including allogeneic (i.e., immuno-nonreactive or “stealth”) cell therapies, including CAR-T, CAR-NK, and engineered mesenchymal stem cell (MSC) cell therapies for regenerative medicine, wound-healing, inflammatory and auto-immune diseases, and tumor-targeting applications.
Our scientists developed a technology that uses mRNA to express both gene-editing proteins and reprogramming factors.
Combined mRNA Gene Editing & Cell Reprogramming is protected by U.S. Patent Number 10,472,611 (with additional patents pending in the U.S. and in other countries). Of note, the granted patent includes claims that are not limited by disease indication, cell type, reprogramming factor(s), mRNA sequence or chemistry, transfection method, target sequence, or type of gene-editing protein.
Example Applications
- Generate gene-corrected personalized cell therapies
- Simplify manufacturing of engineered cell therapies by eliminating serial gene-editing and cell-reprogramming steps
- Take advantage of the clonality of mRNA Cell Reprogramming to generate defined clonal populations of gene-edited cells
- Generate allogeneic pluripotent stem cell-derived CAR-T and CAR-NK cell therapies for the treatment of cancer, and engineered mesenchymal stem cell (MSC) therapies for regenerative medicine, wound-healing, inflammatory and auto-immune diseases, and tumor-targeting applications
Data
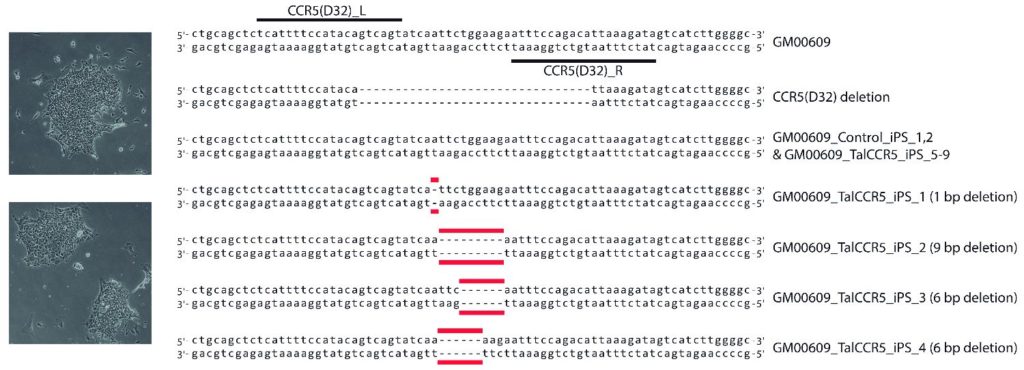
Representative Claim
U.S. Pat. No. 10,472,611
A method for producing a gene-edited, reprogrammed cell, comprising:
(a) providing a non-pluripotent cell;
(b) culturing the non-pluripotent cell; and
(c) transfecting the non-pluripotent cell with one or more synthetic RNA molecules, wherein the one or more synthetic RNA molecules include:
(i) at least one RNA molecule encoding one or more reprogramming factors, and
(ii) at least one RNA molecule encoding one or more gene-editing proteins;
wherein the transfecting results in the cell expressing the one or more reprogramming factors and the one or more gene editing proteins to result in a gene-edited, reprogrammed cell;
wherein step (c) is performed without using irradiated human neonatal fibroblast feeder cells and occurs in the presence of a medium containing ingredients that support reprogramming of the cell.