Cell Reprogramming Medium
Download our Technology Catalog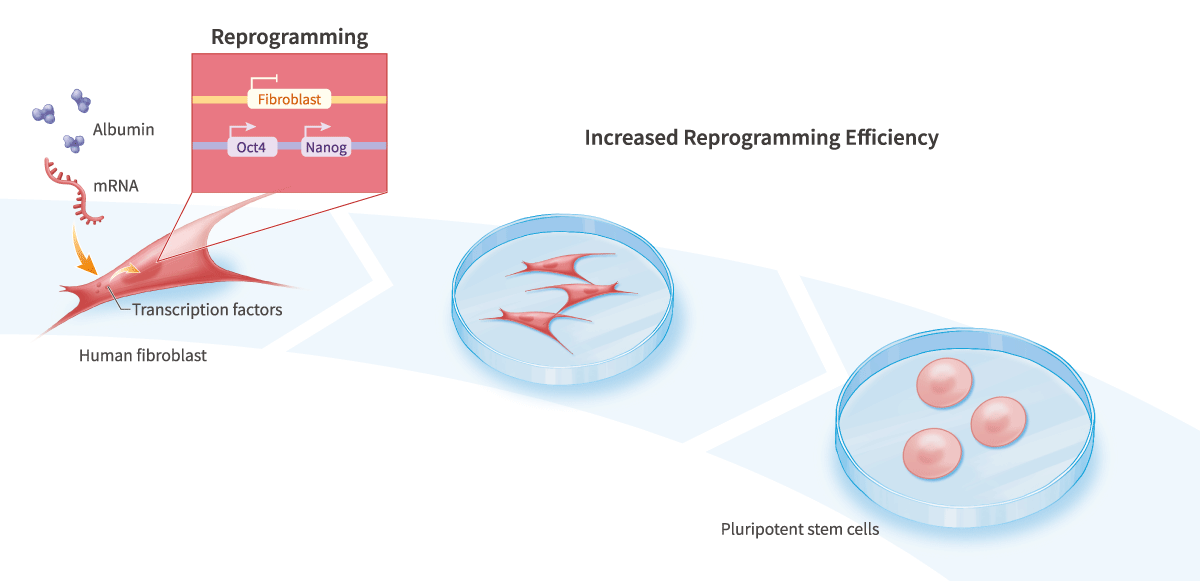
Overview
Conventional cell-culture media, including serum-free and animal component-free media, can result in very low-efficiency cell reprogramming.
Our scientists developed a novel cell-culture medium that can enable dramatically higher efficiency cell reprogramming than conventional media, including when mRNA is used to express reprogramming factors3.
3Harris, J., et al. Mol Ther, Vol 29, No 4S1, 2021
The Cell Reprogramming Medium is protected by U.S. Patent Number 9,127,248, as well as patents in Australia, China, Japan, The Republic of Korea, and Mexico (with additional patents pending in the U.S. and in other countries). Of note, the granted U.S. patent includes claims that are not limited by disease indication, cell type, or method of reprogramming.
Example Applications
- Ultra-high efficiency reprogramming (e.g., reprogram single cells)
- Reprogram without using viruses or other potentially mutagenic vectors
- Reprogram cells quickly, and using a simple protocol (e.g., 4-6 transfections, pick colonies in 8-12 days)
- Reprogram without feeders, conditioning, passaging, immunosuppressants, demethylating agents or other toxic small molecules, pre-mixing or aliquoting of RNA solutions
- Reprogram using a completely animal component-free process
- Use for the development of allogenic or autologous cell therapies
- Combine with Factor’s Chromatin Context-Sensitive Gene-Editing Endonuclease and/or Factor’s Combined mRNA Gene Editing & Cell Reprogramming technology to generate models of genetic disease, gene-corrected patient-specific cell therapies, and allogeneic (i.e., immuno-nonreactive or “stealth”) cell therapies, including allogeneic pluripotent stem cell-derived CAR-T and CAR-NK cell therapies for the treatment of cancer, and engineered mesenchymal stem cell (MSC) therapies for regenerative medicine, wound-healing, inflammatory and auto-immune diseases, and tumor-targeting applications
Data
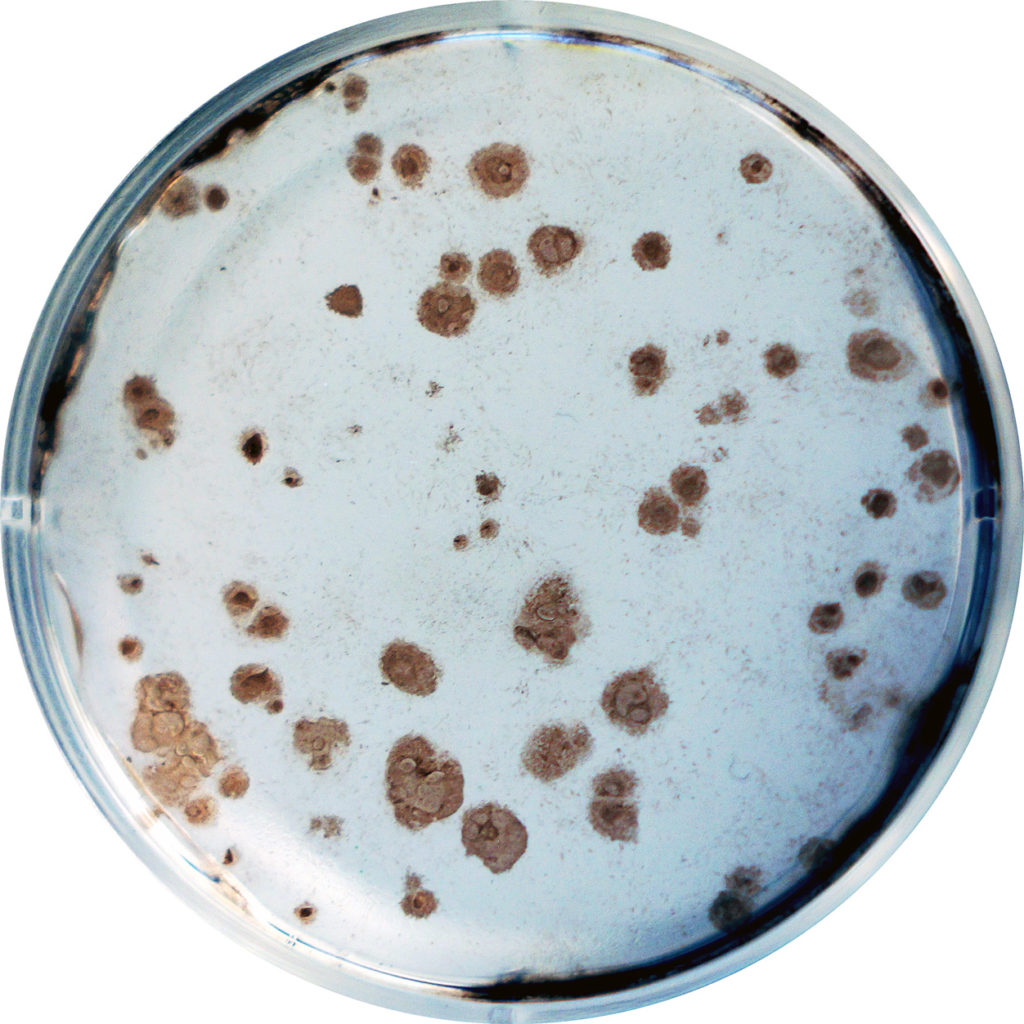
Representative Claim
U.S. Pat. No. 9,127,248
A cell-culture medium comprising: DMEM/F12, 10 μg/mL insulin, 5.5 μg/mL transferrin, 6.7 ng/mL sodium selenite, 20 ng/mL bFGF, and 5 mg/mL albumin, wherein less than 0.65% of the albumin’s dry weight comprises lipids and/or less than 0.35% of the albumin’s dry weight comprises free fatty acids.